AG Robert Slany
Focus of research: AG Robert Slany (opens in a new tab)
The group of Professor Robert Slany is interested in the genetic mechanisms driving cancer development, with a special focus placed on Mixed Lineage Leukemia (MLL).
Prof. Dr. Robert Slany
Lehrstuhl für Genetik (Prof. Nimmerjahn)
- Telefon: +49 9131 85-28527
- E-Mail: robert.slany@fau.de

The HOX -TALE network in normal and leukemic hematopoiesis
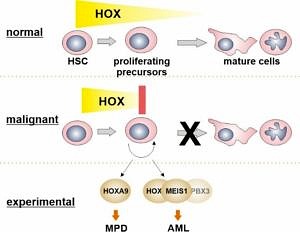
Homeobox transcription factors are important regulators of cellular identity that control cell fate and differentiation during embryogenesis and in adult life.
During hematopoiesis the combined activity of HOX homeobox proteins and their interaction partners of the TALE (three amino acids loop extension) family, in particular MEIS1, determines the size of the rapidly proliferating precursor-cell pool and therefore controls normal steady state repopulation rates. Aberrant presence of HOX/MEIS activity is a strong oncogenic stimulus that is observed in a sizable portion of neoplastic blood diseases with a prevalence in myeloid neoplasms. The ultimate goal of this project is to identify and understand the functional contribution of HOX/MEIS proteins and their downstream mediators towards hematopoietic transformation and to discover potential intervention points that may be amenable for targeted therapy.
Breitinger C., Maethner E., Garcia-Cuellar M.P., and Slany R.K. (2012) The homeodomain region controls the phenotype of HOX-induced murine leukemia. Blood, 120(19):4018-4027
The oncogenic mechanism driving MLL fusion protein mediated leukemia
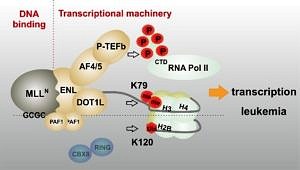
The creation of a MLL fusion protein is the corollary of a chromosomal translocation that fuses the 5’ portion of the gene coding for the histone H3K4 methyltransferase MLL (also called KMT2A) to a wide variety of different partner genes. This creates chimeric proteins that act as untypical transcription factors. MLL fusions recruit parts of the general transcriptional machinery and “lock in” the active status particularly of highly expressed genes.
As a consequence, MLL fusions establish a gene expression pattern that evokes self-renewal and aberrant proliferation in hematopoietic precursor cells, eventually leading to an aggressive leukemia. This MLLr (rearranged) disease is prevalent in infants but it also occurs in up to 10% of all acute leukemia diagnosed in older patients.
Next to the molecular mechanisms driving the physical activity of MLL fusion proteins, we’re especially interested in the downstream effectors driving cellular transformation and here we also look for alterations of cellular metabolism as malleable “hallmark” of cancer.
Garcia-Cuellar MP, Büttner C, Bartenhagen C, Dugas C, Slany R.K. (2016) Leukemogenic MLL-ENL fusions induce alternative chromatin states to drive a functionally dichotomous group of target genes. Cell Reports, Apr 12;15(2):310-22. doi: 10.1016/j.celrep.2016.03.018
Pet project (unfortunately inactive)
Phylogeny of angiosperms in particular within the order Sorbus and the family Burseraceae. That’s what I’d do if I had more time and money…
Publications
A complete and up-to-date list of publications can be found under: http://www.ncbi.nlm.nih.gov/pubmed/?term=slany+r
10 Key Publications:
-
- Hetzner K, Garcia-Cuellar MP, Büttner C, Slany RK. (2018) The interaction of ENL with PAF1 mitigates polycomb silencing and facilitates murine leukemogenesis. Blood 131(6):662-673. doi: 10.1182/blood-2017-11-815035
- Garcia-Cuellar MP, Büttner C, Bartenhagen C, Dugas C, Slany R.K. (2016) Leukemogenic MLL-ENL fusions induce alternative chromatin states to drive a functionally dichotomous group of target genes. Cell Reports, Apr 12;15(2):310-22. doi: 10.1016/j.celrep.2016.03.018
- Bach C., and Slany R.K. (2014) DOTting the Path to Doom: How Acceleration of Histone Methylation Leads to Leukemia. Cancer Cell 8;26(6):781-782.
- Maethner E., Garcia-Cuellar M.P., Breitinger C., Takacova S., Divoky V., Hess J.L., and Slany R.K. (2013) MLL-ENL inhibits polycomb repressive complex 1 to achieve efficient transformation of hematopoietic cells, Cell Reports, May 30;3(5):1553-66
- Breitinger C., Maethner E., Garcia-Cuellar M.P., and Slany R.K. (2012) The homeodomain region controls the phenotype of HOX-induced murine leukemia. Blood, 120(19):4018-4027
- Takacova S., Slany R., Bartkova J., Stranecky V., Dolezel P., Luzna P., Bartek J., Divoky V. (2012) DNA Damage Response and Inflammatory Signaling Limit the MLL-ENL induced Leukemogenesis in vivo, Cancer Cell, 21(4):517-531
- Bach C., Buhl S., Mueller D., García-Cuéllar M.P., Maethner E., and Slany R.K. (2010) Leukemogenic transformation by HOXA-cluster genes, Blood, 115:2910-18
- Müller D., Garcia-Cuellar M.P., Bach C., Buhl S., Mäthner E., and Slany R.K. (2009) Misguided Transcriptional Elongation Causes Mixed Lineage Leukemia, PLOS Biology, 7(11):e1000249 [chosen as research highlight in Nature Genetics 42, 19 (2010)]
- Müller D., Bach C., Zeisig D., Garcia-Cuellar M.P., Monroe S., Sreekumar A., Zhou R., Nesvizhskii A., Chinnaiyan A., Hess J.L., and Slany R.K. (2007) A Role for the MLL Fusion Partner ENL in Transcriptional Elongation and Chromatin Modification, Blood, 110: 4445-4454
- Zeisig B.B., Milne T., Garcia-Cuellar M.P., Schreiner S., Martin M.E., Fuchs U., Borkhardt A., Chanda S., Walker J., Soden R., Hess J.L., and Slany R.K. (2004) Hoxa9 and Meis1 are key targets for MLL-ENL mediated cellular immortalization. Mol. Cell. Biol. : 24, 617-628
Click here for the full list of publications
Funding
Research of the Slany group is funded in the context of the following projects:
- DFG: Deciphering the oncogenic MLL network (2017-)
- Deutsche Krebshilfe: The function of the homeobox transcription factor Meis1 in the etiology of acute myeloid leukemia
