AG Falk Nimmerjahn
Focus of research: AG Falk Nimmerjahn
The group of Professor Falk Nimmerjahn is interested in antibody-mediated effector functions and mechanisms of humoral tolerance.
Prof. Dr. Falk Nimmerjahn
Lehrstuhl für Genetik (Prof. Nimmerjahn)
- Telefon: +49 9131 85-25050
- E-Mail: falk.nimmerjahn@fau.de
<< Staff of the Nimmerjahn group >>

Molecular and cellular pathways underlying autoimmunity
Immune responses and the cells involved are regulated through a variety of activating and inhibitory signals. Marginal discrepancies in this balance can lead to either too weak or too diligent immune responses. This dysregulation can have severe consequences, such as uncontrolled infections with pathogenic microorganisms on the one hand, or the initiation of auto immune diseases on the other.
Antibodies play important roles in both scenarios, as they are involved both in the bodies immune defense as well as the destruction of healthy tissue, in the context of autoimmunity. Therefore, the production of antibodies in the body is tightly controlled at multiple checkpoints (Fig. 1).
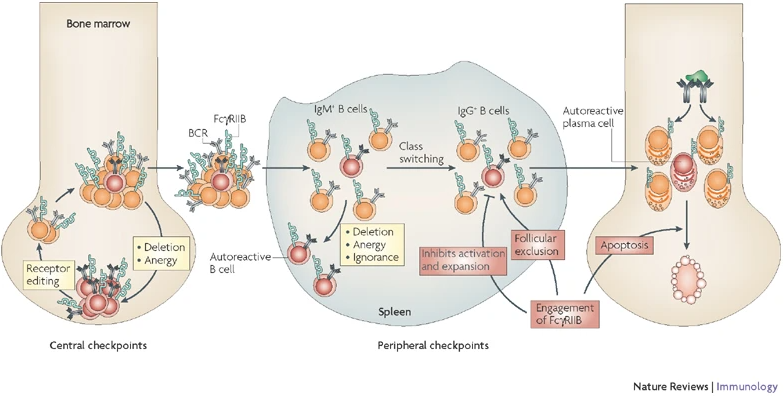
An important factor herein is the regulation of signals stemming from the BCR. Lack of several proteins has been associated with dysregulation of the B cell response. In our previous studies, we could show, that the inhibitory Fc receptor FcgRIIB acts as a crucial checkpoint in late B cell developmental stages.
Studies on SLE patients, combined with our own results of work on CIDP (chronic immune-mediated demyelinating polyneuropathy) patients, have underlined the importance of this negative regulation also in the human context. Beside classic mouse models, we also utilize novel humanized mouse models, which allow us to assess the translatability of our results to the human immune system.
Effector mechanisms of antibodies in mice and men
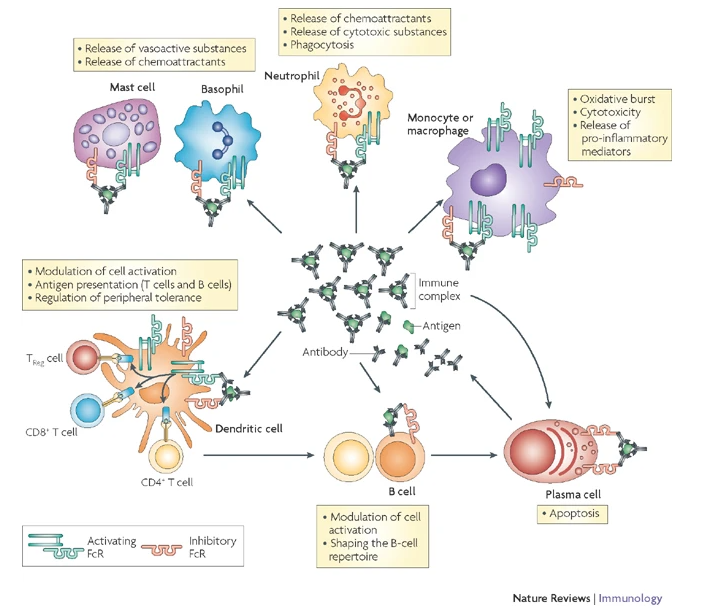
The starting point of our studies has been the observation, that certain IgG subclasses are more capable of mediating the destruction of healthy tissues in the context of auto immune disease, and tumor cells in the context of immunotherapy. We could show that functional inactivation of all murine Fc receptors abrogates activity of all IgG subclasses in vivo. During our studies, we identified FcgRIV as a novel activating Fc receptor predominantly expressed on neutrophils and monocytes, that specifically binds IgG2a and IgG2b.
For the first time, we managed to map the functional activity of all IgG subclasses to their respective activating Fc receptors.
In follow-up studies, these insights could be translated onto other model systems, such as e.g. therapeutically relevant tumor models. Those insights are not only of great importance for the murine system, but are also of value for the human immune system, since FcgRIV could be identified as the then unknown orthologue to human FcgRIIIa, a receptor that plays integral roles in effector functions of therapeutic antibodies.
On the foundation of this preliminary data, we are currently trying to identify cell populations, that are responsible for IgG/Fc receptor mediated effector functions (Fig. 2).
The influence of glycosylation variants on antibody function
Another focus of our research lies in the functional analysis of antibody glycosylation variants. Each IgG molecule consists, beside the protein backbone, of a sugar chain, which itself is made up of a central lattice and a variable number of terminal and branched sugar residues such as fucose, sialic acid or galactose. Beside their long-known function in ensuring correct antibody folding, these glycosylation variants also heavily impact antibody functionality.
This has big implications for the usage of such antibody variants in the therapy of malignant diseases, but also for the use of anti-inflammatory IgG preparations in the context of auto immune diseases (IVIg-therapy). We try to assess this IgG-mediated anti-inflammatory mechanism in a variety of projects.
Publications
Falk Nimmerjahn on Google Scholar
5 Key Publications
- Lehmann, B., M. Biburger, C. Brückner, A. Ipsen-Escobedo, S. Gordan, C. Lehmann, D. Voehringer, T. Winkler, N. Schaft, D. Dudziak, H. Sirbu, G.F. Weber, and F. Nimmerjahn. 2017. Tumor location determines tissue-specific recruitmetn of tumor-associated macrophages and antibody-dependent immunotherapy response. Science Immunol 2:1-11.
- Kao, D., H. Danzer, M. Collin, A. Gross, J. Eichler, J. Stambuk, G. Lauc, A. Lux, and F. Nimmerjahn. 2015. A Monosaccharide Residue Is Sufficient to Maintain Mouse and Human IgG Subclass Activity and Directs IgG Effector Functions to Cellular Fc Receptors. Cell Rep 13:2376-2385.
- Nimmerjahn, F., S. Gordan, and A. Lux. 2015. FcgammaR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol 36:325-336.
- Schwab, I., A. Lux, and F. Nimmerjahn. 2015. Pathways Responsible for Human Autoantibody and Therapeutic Intravenous IgG Activity in Humanized Mice. Cell Rep 13:610-620.
- Biburger, M., S. Aschermann, I. Schwab, A. Lux, H. Albert, H. Danzer, M. Woigk, D. Dudziak, and F. Nimmerjahn. 2011. Monocyte subsets responsible for immunoglobulin G-dependent effector functions in vivo. Immunity 35:932-944.
Click here for the full list of publications
Funding
Research of the Nimmerjahn group is funded in the context of the following projects:
- DFG TRR130/P13: Understanding the role of the human inhibitory FcR for autoreactive and protective humoral immune responses in vivo (2017-2021)
- DFG SFB1181/A07: Investigating the molecular and cellular pathways of intravenous immunoglobulin G mediated resolution of established autoimmune inflammation (2019-2024)
- DFG FOR2953: Role of α2,6-linked sialic acids on B cell development and antibody function (2019-2022)
- DFG D-A-CH: Human IgG Subclass Glycosylation (2019-2021)
- DFG FOR2886/B2: IgG glycosylation as a regulative factor controlling onset of RA (2019-2021)
- DFG LU877/19-1: Pathogenicity of IgA antibodies in pemphigoid disease (2020-2022)
- NIH U19 Centre for Excellence in Translational Research: Consortium for Immunotherapeutics against Emerging Viral Threats (2018-2023)
- NIH RFA-AI-18-042: Fc-dependent mechanisms of antibody-mediated killing (2019-2024)
